Manufacturing License
Steps to get a Manufacturing License
Step-1 Register your Firm with CDSCO
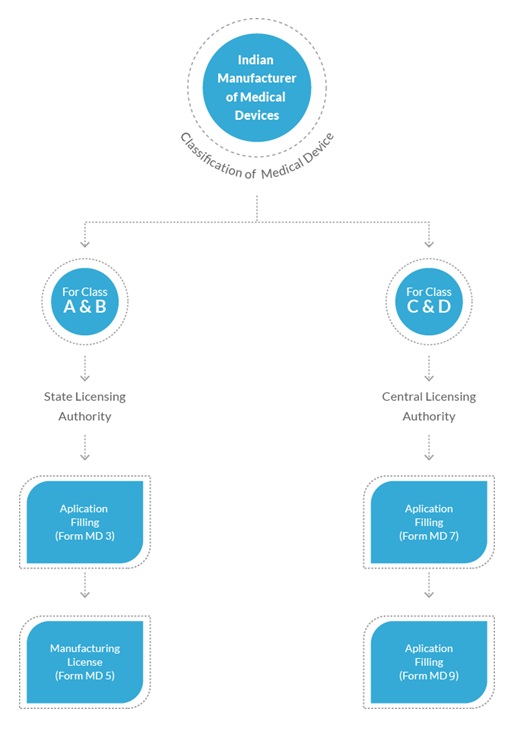
Step-2 Apply for Manufacturing License of Class A&B in Form MD-3 and for Class C&D in MD-7
Step-3 Upload the following documents for the License on CDSCO portal
- Cover Note(with payment details)
- Plant OR Site Master File
- Device Master Files for all the Devices
- Undertaking of site compliance.
- Test License
- Performance Evaluation Report
- Constitution of the Firm
- Ownership documents/ rent agreement for the Plant.
- Label and IFU Of the Devices.
Step-4 Make payment CDSCO Fees ONLINE to SLA
Step-5 Upload your challan of the payment
Step-6 Get the legal document- Sign it digitally and upload.
Fees CDSCO
Site License Fees- Rs 5,000 Class A&B Device and Rs 50,000 for Class C&D Devices
Each distinct Device fees- Rs 500 for Class A&B Devices and Rs 1000 for Class C&D Devices
Total CDSCO Fees to be paid to State Drug Controller for Class A& B Devices and to CSCO Directly for Class C&D Devices
Against Form MD-3- Medical Devices Licensing
Fee can be paid through Government Treasury challan, under Head of Account- 0210- 04-800-02-00 Other receipts
Department Name: Drugs Controller, Rajasthan, Jaipur
Office Name: Asstt. Drugs Controller, NDNP, Jaipur, Rajasthan
Consultancy Fees
Creating AC with CDSCO- Rs 15,000+GST@18%
15,000+ 18% GST per Device Family
Discounts applicable as 10% for 2-5 Device, 15% 3-10 Devices, 30% on more than 10 Devices
Fees to be paid before uploading the documents.
Scope of consultancy
- Drafting Plant Master File
- Drafting Device Files for each group
- Drafting Essential Principal Check List for each group.
- Drafting performance Evaluation reports
- Drafting declaration for Test License.
- Drafting undertaking for Site compliance
- Filling up form MD-3 / MD-7 with all details.
- Examining and Uploading all the documents.
- Drafting cover letter for submission.
- Any other document required for submission.
- Representing to CDSCO for any queries.

Way forward: The following are steps to follow
- Submit the fee as described above through the challan. Contact the office as detailed above for fees submission.
- Send the details of the Fees submitted with transaction receipt and challan details.
- Send the user ID and password for the registration which you have done.
- Fill up the Dimensions- Packed and Unpacked for all the families of products. You can provide a range of dimensions for Length, Breath and Height in cm/inches etc. ; Storage Temperature and materials of construction for the devices.
- Prepare Instruction for use for all the devices- Single/DOUBLE page with details of parts and usage instructions. Where ever it is not practical to make the IFU please details at least the parts of the devices or a full page catalogue for the individual devices.
- For Plant master file- Provide the Factory Map, Details of Raw material storage area, Shop Floor, Finished Goods storage area, Testing areas if any, List of Instruments and Equipment for manufacturing with calibration status, Organogram with Names and Details of Qualifications of Managing Director, Plant Manager, Quality Manager, Management Representative, Customer Relations officer, Administration, HR and Accounts manager. You can duplicate the function of key managerial staffs, drainage system, drinking water , electricity layout and person hygiene and personal protection equipment, first aid facility etc.
- For device files please submit any data / reports you have on testing of the devices/ raw materials and any BIS Standard.
Exempted Class A (Non-Sterile and Non-Measuring Devices)
Most of the Class A devices can now be manufactured by simple undertakings to be uploaded into the CDSCO AC on the Portal.
These devices consist of the following:
- All hospital Furniture including hospital beds, mattresses, Chairs, Tables, Lockers etc.
- Reusable Surgical Instruments.
- Bronchoscopes, Laryngoscopes etc.
- Surgical Cameras.
- Bio Feed back Devices
- X-Ray View Box
- PPE Equipment (Non-Sterile)- Gowns, Gloves etc.
- Physical Support Equipment, rehab and assisted devices including wheelchair, walker etc.
- All Class A Ophthalmic Devices.
- Class A ENT Devices
- All other Class A Devices of Dental and other departments.
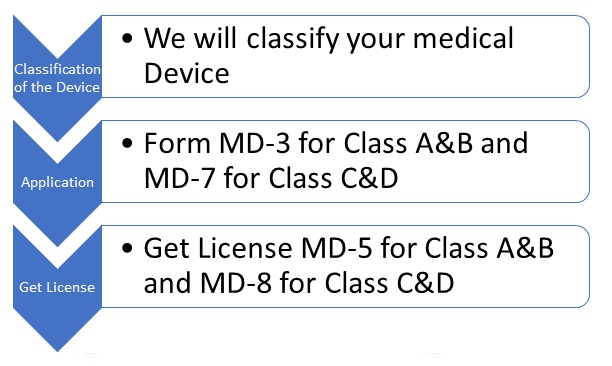
We will help you in the Entire Manufacturing License Process Chain as below:
MEDICAL DEVICE – FOR MANUFACTURERS
Pertaining to the New Medical Rules 2017, all medical devices have been classified into four different categories. Namely, Class A, Class B, Class C and Class D. Any company which intends to manufacture these devices for sale or distribution must apply for a manufacturer’s license with CDSCO.
Class A and Class B devices are considered as low risk and moderate risk devices. The application for manufacturing medical devices falling under these classes has to be filed with the State Licensing Authority. Whereas, Class C and Class D devices are classified as High and Very High Risk devices. The application for manufacturing these has to filed with the Central Licensing Authority.
CDSCO has also defined different application fees for each category of medical devices. Our professionals at TMSCPL have the right knowledge, skill set and experience to help you with filing an application for obtaining manufacturers license. Our experts help you in reducing hassle and expediting your application process.
Permission to Manufacture or Permission for loan license to manufacture Class A & B Medical Device in India from State FDA (Form MD-5 and Form MD-6)
Class A and Class B devices are classified as low risk and moderate risk devices. The License for manufacturing these devices can be applied and obtained at the State Licensing Authority. The application is made using form MD-5 whereas the permission is granted through form MD-6.
TMSCPL has presence across all the states in India to help you with filing an application with your respective state licensing authority. Our experts will take the pain off your head by helping you create and file the application. We offer end-to-end customer support for our clients.
Permission to Manufacture or Permission for loan license to manufacture Class C & D Medical Device in India from CDSCO (Form MD-9, Form MD-10)
Class C and Class D medical devices are classified as High Risk to Very-High Risk medical devices. The application procedure for these devices is more stringent and complex as compared to Class A and B devices. The application for the manufacturer license is filed at the Central Licensing Authority.
Filing application for these devices is a hectic and complex procedure. Our team at TMSCPL helps simplify and expedite this process through regular follow up meeting and proper submission of documents.
Permission for test license to manufacture Medical Device (Form MD-12, Form MD-13)
Manufacturers may at times require license for production of medical devices intended for the purpose of testing, training, clinical evaluation or demonstrations. For this an appropriate test license has to be obtained by the Central Licensing Authority for manufacturing such devices. The test license can be obtained for any class of medical devices.
Our technical team at TMSCPL helps such clients by easing the process of filing the application and obtaining the test license.
Do you need assistance with application and licensing?
With TMSCPL, you will receive expert advice, 24 hour support, practical guidance and complete technical assistance right from the beginning till the very end of your penetration into the Indian market.